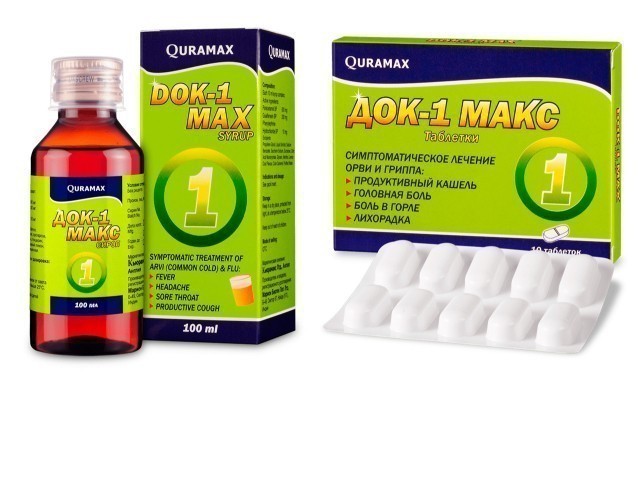
The Dok-1 Max drug that entered Uzbekistan was found to contain toxic substances. There are traces of crime in the case
Crime
−
27 December 2022 42291 3 minutes
Following the death of 18 children in Samarkand due to consumption of Dok-1 Max syrup, 7 officials were dismissed. It was determined that there were indications of a crime in this case, and the documents were sent to the law enforcement authorities. This was reported by the Ministry of Health of the Republic of Uzbekistan.
It is noted that in connection with this situation, a service inspection was conducted by the members of the special working group based on the relevant order of the Ministry of Health.
India’s Marion Biotech Pvt. Dok-1 Max tablets and syrup, which belongs to the company “Dok-1 Max” was registered in Uzbekistan in 2012 and was put on sale this year. According to the information from the Pharmaceutical Network Development agency, each series of drugs has been checked and a certificate of conformity has been issued to them. This drug was imported into the territory of Uzbekistan by “Kuramax Medical” Limited Liability Company.
During the investigation, 18 out of 21 children with acute respiratory diseases died after drinking Dok-1 Max syrup.
“The aforementioned children took this drug in combination with other drugs at home for 2-7 days, up to 2.5-5 milliliters 3-4 times a day. Naturally, this amount is much more than the norm for a child.
All the children were given the drug without a doctor’s prescription. Since the main ingredient in the drug is paracetamol, Dok-1 Max syrup was incorrectly used by parents as an anti-cold remedy independently or on the recommendation of pharmacists. This caused the condition of patients to worsen.
However, paracetamol should be given in a dose of 100-125 milligrams to a child up to 1 year old, 200 milligrams to a child from 1 to 3 years old, and 250 milligrams to a child from 3 to 5 years old, 1 or 2 times a day only when the body temperature is above 38-38.5 degrees. “It is strictly forbidden to use this medicine when the body temperature is normal,” the Ministry of Health said.
In addition, preliminary laboratory studies have shown the presence of ethylene glycol in this series of Dok-1 Max syrup. This substance is toxic, and about 1-2 ml/kg of a 95% concentrated solution causes serious changes in the patient’s health, namely vomiting, fainting, seizures, problems with the cardiovascular system and acute kidney failure.
Taking into account all cases of violation of the law found during the study of the working group of the Ministry, 7 people are responsible for being careless and inattentive to their duty, neglecting to conduct an analysis of the childrens deaths on time and not taking the necessary measures. The employee was released from his position and disciplinary measures were applied to a number of specialists. All collected documents were sent to law enforcement agencies.
At the moment, Dok-1 Max tablets and syrup drugs have been withdrawn from sale in all pharmacies in the country in the established order. The shortcomings identified on the basis of study materials, the issue of the responsibility of medical workers will be considered at a separate board meeting of the Ministry of Health.
Live
All